Lewis And Dot Structure Of (NH4) 2SO3, Al2(SO4) 3 And Fe(ClO4) 3
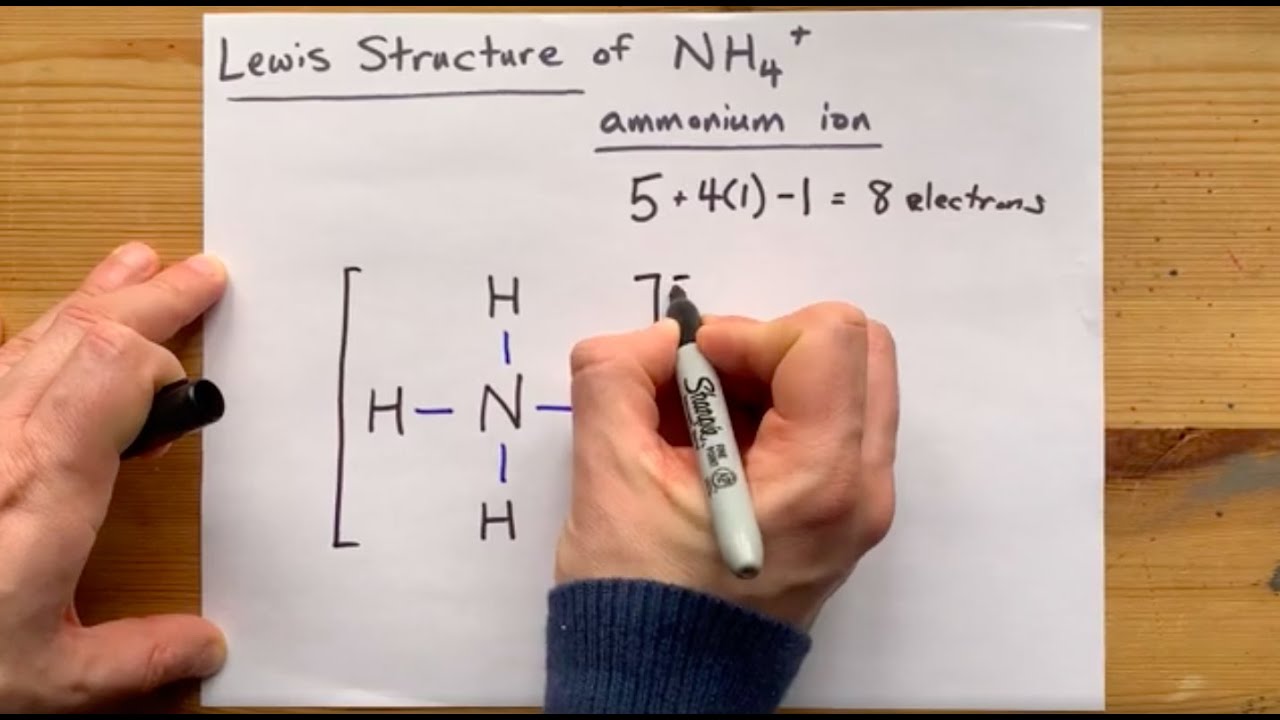
A step-by-step explanation of how to draw the NH4+ Lewis Dot Structure (Ammonium ion).For the NH4+ structure use the periodic table to find the total number.

NH4+ Molecular Geometry / Shape and Bond Angles YouTube
Lewis Structure Finder Added Jun 9, 2014 by WebTester in Chemistry This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

NH4+ Lewis Structure How to Draw the Dot Structure for NH4+ (Ammonium
113 7.2K views 2 years ago A brief explanation for how to write the Lewis Dot Structure for the Ammonium Ion including it's molecular geometry and bond angles..more.more 4.3M views 6 years.

NH4+ Lewis Structure Lewis Dot Structure for NH4+ Ammonium ion
Step-1: NH4+ Lewis dot Structure by counting valence electrons on the nitrogen atom Step-2: Lewis Structure of NH4+ for counting valence electrons around the terminal hydrogen atom Step-3: Lewis dot Structure for NH4+ generated from step-1 and step-2 How to calculate the formal charge on a nitrogen and hydrogen atoms in NH4+ Lewis Structure?
Solved Draw the Lewis structure for NH4 Draw the Lewis dot
Let's draw and understand this lewis dot structure step by step. (Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of NH4+ ion). Lewis Dot Structure for NH4+ (Ammonium ion) Watch on 6 Steps to Draw the Lewis Structure of NH4+

Best Overview NH4+ Molecular Geometry Science Education and Tutorials
Draw the Lewis Dot Structure for the following cation:NH 4+. A B C D 2795 1 Previous problem Next problem 4:28m Watch next Master Lewis Dot Structures: Ions with a bite sized video explanation from Jules Bruno Start learning Comments (0) Question 53 Textbook Question (b) With what allotrope of oxygen is it isoelectronic? 280 Question 60a
Lewis Structure of NH4+, Ammonium ion YouTube
Summary References Contributors and Attributions Learning Objectives To draw Lewis Structures for molecules and polyatomic ions with one central atom. Introduction to Lewis structures A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule.
Chem Filling in the Valence Electrons of an Electron Dot Structure
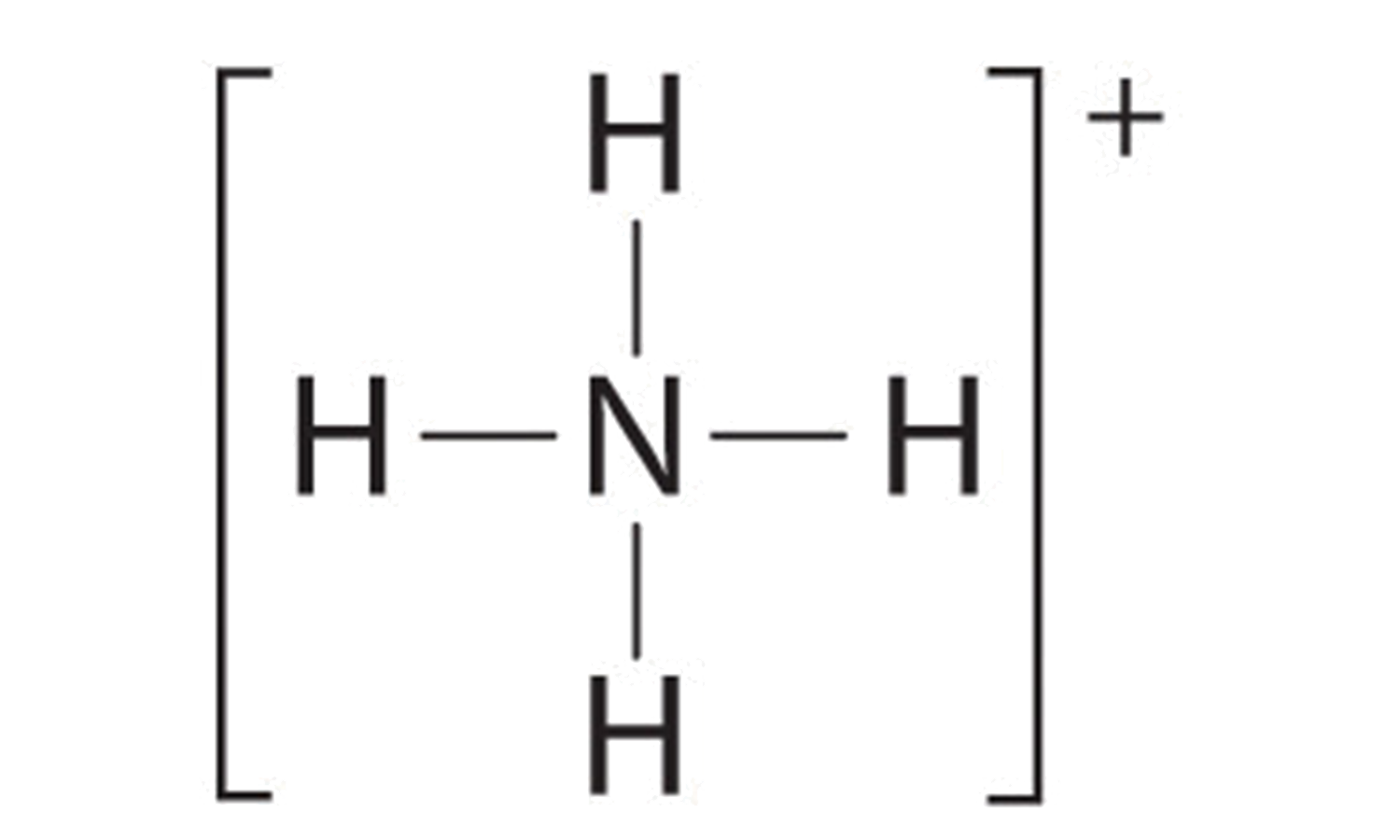
By Biswarup Chandra Dey The NH4 Lewis structure refers to the arrangement of atoms and electrons in the ammonium ion. Ammonium, with the chemical formula NH4+, is a positively charged polyatomic ion composed of one nitrogen atom bonded to four hydrogen atoms.

NH4OH Lewis Dot Structure (Ammonium Hydroxide) YouTube
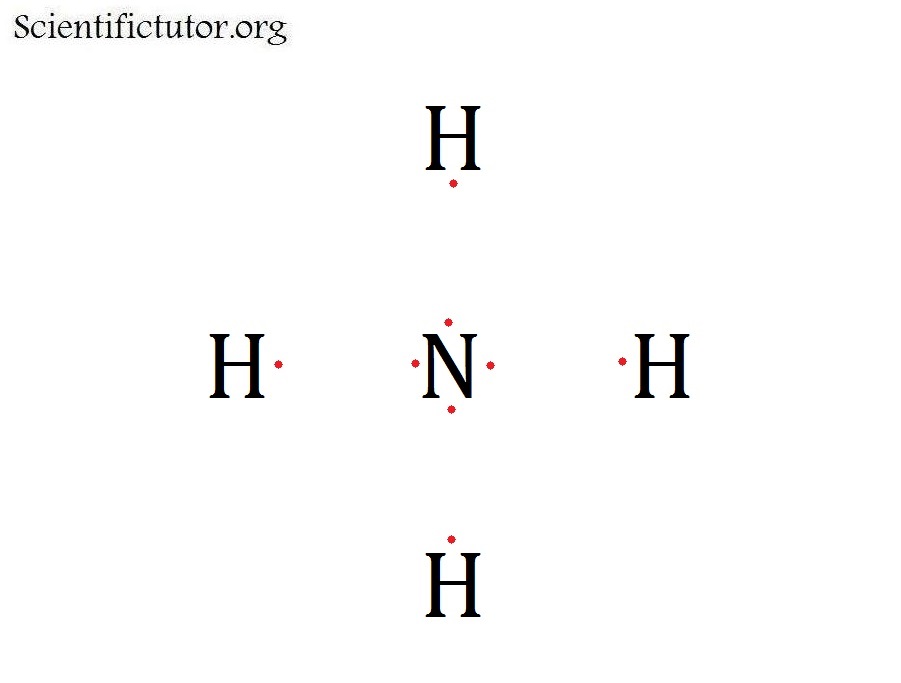
Draw the skeleton structure Now we can draw the skeleton structure of the NH4+ ion. Place the nitrogen atom in the center and surround it with the four hydrogen atoms. Each hydrogen atom will form a single bond with the nitrogen atom. 4. Distribute electrons Next, distribute the remaining electrons to satisfy the octet rule.

draw the Lewis dot structure of the following molecules (1) H2O (2)NH4
Lewis Structure is a simplified arrangement and presentation of the electrons present in the valence shell of a molecule. A Lewis Structure is a depiction of the arrangement of electrons in the standalone atoms of an element. In the Lewis Structure, electrons are depicted as dots.

How to draw NH4+ Lewis Structure? Science Education and Tutorials
Let's do the Lewis structure for NH4+, the ammonium ion. So Nitrogen, on the periodic table, is in group 5, so it has 5 valence electrons. Hydrogen, group 1; we've got 4 of these, though; four Hydrogens, so let's multiply that times 4. And if you see a plus sign, that means you've lost a valence electron. So we've lost one, that's minus one.
Which Of The Following Represents The Lewis Dot Diagram Of Ammonia Nh3
Drawing dot structures Drawing Lewis diagrams Worked example: Lewis diagram of formaldehyde (CH₂O) Worked example: Lewis diagram of the cyanide ion (CN⁻) Worked example: Lewis diagram of xenon difluoride (XeF₂) Exceptions to the octet rule Counting valence electrons Lewis diagrams Resonance Resonance and dot structures Formal charge
Nh4 Dot Structure
NH4+ Lewis Structure - Ammonium Ion The Organic Chemistry Tutor 7.28M subscribers Join Subscribe Subscribed 697 92K views 3 years ago This chemistry video tutorial explains how to draw the.

NH4+ Lewis Structure Ammonium Ion YouTube
Steps of drawing the lewis structure of NH 4+ are explained in this tutorial. NH 4+ lewis structure You see, there are four hydrogen atoms around nitrogen atom. Therefore, nitrogen atom is the center atom. Also, there is a +1 charge on nitrogen atom. Steps of drawing lewis structure of NH 4+

draw electron dot structure for ammonium NH4 Science Carbon and its
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.

Equation For Reaction Of Ammonium Ion With Water Tessshebaylo
Custom Search Lewis Dot of the Ammonium Ion NH 4+ Back 70 More Lewis Dot Structures Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Ammonium has a tetrahedral shape with bond angles of 109.5 o.
